24+ Periodic Table With Shells
8 electrons occupy the second shell. Elements in group 1 and group 2 are metals.

A Roadmap For Mechanically Interlocked Molecular Junctions At Nanoscale Acs Applied Nano Materials
1 2 and 3.

. In this table you can see that helium has a full valence shell with two electrons in its first and only 1n shell. For elements in groups. All the Group 1 elements.
The ions have the electronic structure of a noble gas group 0 element with a full outer shell. This electronic configuration can be written as 281 each dot. Number of electrons in outermost shell.
Now each orbital can fit two electrons. The number of electrons. What is the.
The number of subatomic particles in an atom can be calculated from the atoms atomic. The s- p- and d-block elements of the periodic table are arranged into 18 numbered columns or groups. 2 electrons occupy the first shell.
The electrons in an atom are arranged in shells that surround the nucleus with each successive shell being farther from the nucleus. Khan Academy is a nonprofit with the mission of. Electron shells consist of one or more subshells and.
Despite having a 1s 2 configuration with two valence electrons and thus having some similarities with the alkaline earth metals with their ns 2 valence configurations its. 1 electron occupies the third shell. And so 11 electrons.
Atomic structure and the periodic table. So if youre thinking about the subshell the s subshell could fit two electrons the p subshell can fit six electrons the d subshell can fit 10 electrons. Electronic configuration feature Link to the periodic table.
Number or numbers of circles. Learn for free about math art computer programming economics physics chemistry biology medicine finance history and more. Similarly neon has a complete outer 2n shell containing eight electrons.
The information shown is for elements with atomic numbers 1 to 20. Atoms of group 1 elements have one electron in their outer shell and atoms of group 2 elements have. Below is a table showing the maximum number of electrons an element can have for each of its energy level shells.
Helium is an exception. Atoms consist of a nucleus containing protons and neutrons surrounded by electrons in shells. Rows in the periodic table periods.
Sulfur is in group 6 of the periodic table. The elements in each group have the same number of valence. Remember each black orbital represents a shell first shall all the way to the seventh shell here will realize here that the shells of an atom are directly related to the periods or row of the.
In the outer shell of all the elements in a group is the same as the group number.
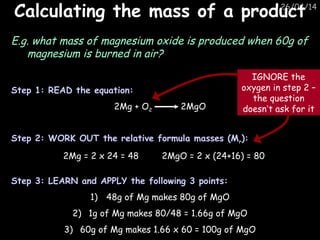
Aqa Chemistry C2 Revision
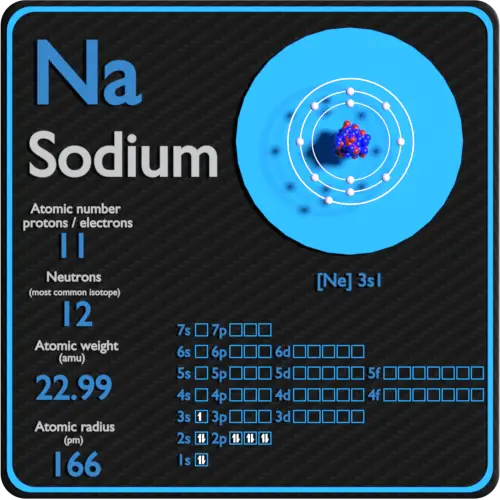
Sodium Protons Neutrons Electrons Electron Configuration

Printable Periodic Table Pdf With Shells
Ppt Ks4 Chemistry Powerpoint Presentation Free Download Id 6054682

File Periodic Table Of Elements Showing Electron Shells Svg Wikimedia Commons

Analytical Solution For Segmental Tunnel Lining Incorporating Interaction Between Adjacent Rings Journal Of Engineering Mechanics Vol 146 No 7
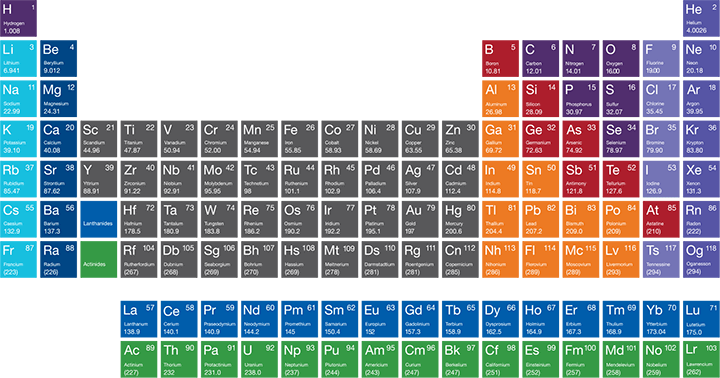
Interactive Periodic Table Of Elements

Periodic Table Of The Chemical Elements Showing In Full Blue Squares Download Scientific Diagram
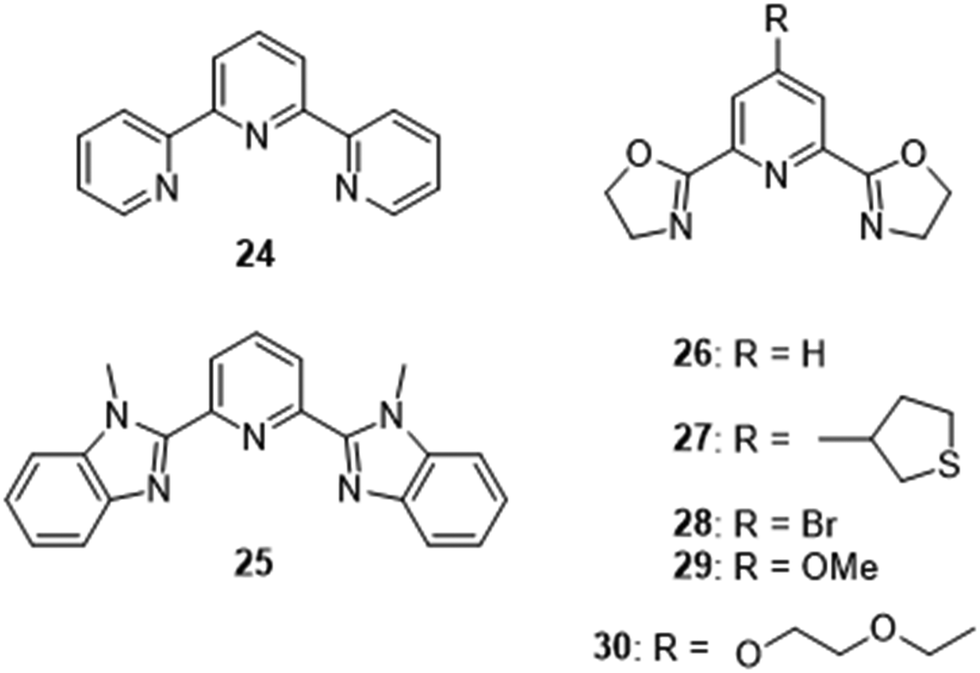
Lanthanide Directed Synthesis Of Luminescent Self Assembly Supramolecular Structures And Mechanically Bonded Systems From Acyclic Coordinating Organic Chemical Society Reviews Rsc Publishing Doi 10 1039 C6cs00116e

3 Periodic Table And Electron Shells Flux Science

Publications Schalley Group Department Of Biology Chemistry Pharmacy

Color Periodic Table With Shells

Smallest Part Of An Element With All The Properties Of That Element 2 Parts Ppt Download

Printable Periodic Table Pdf With Shells

Black And White Periodic Table With Electron Shells Png Image Transparent Png Free Download On Seekpng

Alternative Periodic Tables Chemistry Group Chemical Element Colorful Elements Furniture Spiral Png Pngegg
Electron Shells And The Periodic Table Igcse Chemistry Youtube